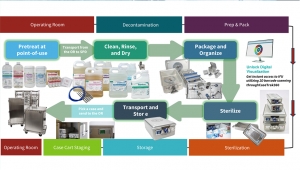
Rethinking Sterile Processing
Healthcare no longer operates in yesterday’s world. The Industrial Revolution has given way to the Digital Revolution, transforming how we diagnose, treat, and operate. Minimally invasive procedures now rely on sophisticated endoscopes and robotic instruments — devices that demand meticulous, step-by-step reprocessing and strict adherence to IFUs to ensure safety, performance, and longevity.
The KISS Principle for Instrument Processing
The last few blogs focused on the importance of standardization to decrease waste, remove unnecessary steps, improve turnover, and provide consistent workflow and increase efficiency. Currently, standardization and offsite processing are key buzz words for strategic plans to reduce handling and cut costs in SPD.
Standardization: The Missing Link in OR–SPD Communication
This week at the OR Leadership Summit, Case Medical connected with OR leaders to explore what it truly takes to achieve smoother operations, clearer communication, and reliable compliance. When communication breaks down between the operating room and sterile processing, the symptoms are easy to spot—late trays, incomplete sets, urgent phone calls, and last-minute workarounds.
Driving Better Outcomes Through Healthcare Standardization
Standardization can improve productivity, reduce costs, and create supply chain efficiency. Healthcare facilities are looking at the Total Cost of Ownership to reduce waste and have what is needed in stock, even during challenging times and supply chain shortages.
Penny Wise and Pound Foolish
When budgets are tight, people sometimes look for quick fixes to save on immediate costs. Unfortunately, they will end up being sorry if what looks like a less expensive product costs more in the long run.
Keep Cleaning with Soap and Water
Cleaning helps prevent infection by physically removing germs, dirt, and other debris from surfaces and objects. This removal significantly reduces the number of germs available to spread, making it less likely that individuals wi
A Formula for Success for SPD
We just returned from the HSPA Conference in Louisville Kentucky and learned a few things that can be improved for safe and effective medical device reprocessing. One that is top of mind was water quality...
Sustainability and Healthcare Go Hand in Hand
This weekend, both of my coffee pots died. They weren’t cheap. They ground the coffee beans and even made cappuccino if we used the frothier. Both were the same popular brand purchased approximately three years ago...
Don't blame my baby: It could be your reused water
At Case Medical, we recycle the water we use for production purposes like anodizing and passivating our metal containers, trays, and metal component parts...
Filtration: Water Treatment Without Harm
Nature works to filter and release water over time – for free! Soil layers, wetlands, and even the root systems of plants like mangroves act as natural filters, removing pollutants and sediments...