SPD Can Prevent Prion Diseases from Spreading
The central role of the Sterile Processing Department (SPD) is to decontaminate surgical instruments for patient care procedures. By carefully following cleaning and sterilization protocols for used devices, the SPD plays a critical role in infection prevention. However, not all infectious agents are created equal. Some are highly resistant, like infectious prion proteins which are not only persistent, but attach themselves to stainless steel devices used for patient procedures. Inadequate decontamination methods, in general, can lead to infection, illness, and sometimes fatal diseases in patients. Prion diseases are a group of progressive, fatal neurological diseases that occur when prion proteins, which are found on the surface of every cell, begin to misfold and form clumps in the brain. This eventually causes irreversible brain damage and death, but no one will know as the disease progresses over decades. Diseased prion proteins easily adhere to stainless steel instruments used in surgical procedures. This is where the action that SPD takes in disease prevention becomes so important.

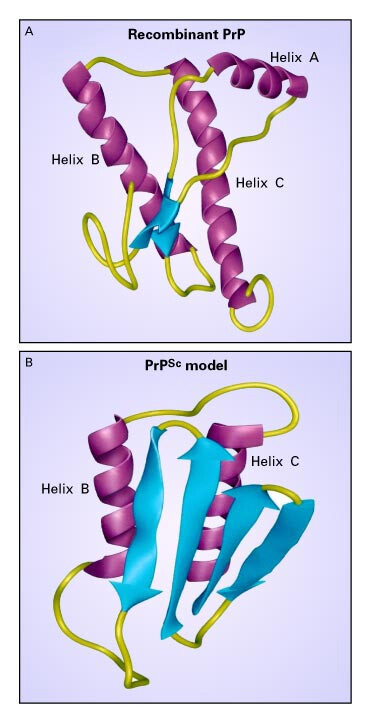
Understanding the Risks in Healthcare Settings
Hospitals concerned with potential exposure to Creutzfeldt-Jacob Disease (CJD) often take extreme measures from treating instruments with heavy doses of bleach to incinerating anything that might have come in contact with the patient. The reaction is warranted given the risks. However, we, at Case Medical, have developed a better approach to the problem which may not be a cure, but can be more than an ounce of prevention. CJD, which is the most common prion related disease in humans, can be caused by exposure to contaminated devices. Once infected, it can take years, often decades, before patients start to show symptoms. Common symptoms include imbalance and incoordination, memory loss, impaired thinking and psychiatric symptoms such as depression and anxiety. Upon onset of symptoms, CJD can progress very quickly and prove to be fatal within a year. Infected patients can transmit the disease while asymptomatic, making detection nearly impossible. CJD can only be confirmed postmortem or with a brain biopsy, which rarely occurs. While prion diseases can occur for different reasons including consumption of infected meat, inherited predisposition, the more relevant risk for humans is in a healthcare setting via contaminated instruments.
Prevention is the Key: Separate, Isolate, Decontaminate
Most healthcare facilities already separate eye instruments from other devices. Given the risk to patients of infectious prion proteins, let’s consider why they should be separated from other devices and decontaminated separately. We highly recommend taking a preventative, proactive approach to separate and isolate suspected devices for special treatment prior to sterilization. Erring on the side of caution, assume that a patient undergoing a cranial or spinal procedure with suspected neurological symptoms could be a carrier. Doesn’t it make sense to isolate the used devices and treat them separately? Eye instrumentation is separated for sonication and decontamination for reasons including preserving the delicate instrumentation and possibly to avoid TASS. Proactive prevention should therefore be exercised while processing instruments when there are suspected cases of CJD. A pre-treatment method based on science and years of research is not a cure for the disease, but can reduce the risk of cross-contamination. Surgical instruments and medical devices that have been potentially exposed to prion diseases can be separated and isolated from other instruments processed in the SPD.

BioGone – A Better Way to Decontaminate
Case Medical holds a patent for a decontamination method to handle potential prion contaminated devices. A peer-reviewed paper in the Association for the Advancement of Medical Instrumentation’s (AAMI) Biomedical Instrumentation & Technology (BI&T), and an article in HSPA's Vendor Vantage provide a description of the process and the solution. The documents share results from years of research on BioGone, an enzymatic detergent from Case Medical, along with a specific, validated process, to control the critical variables of contact time, water quality, mechanical action, and temperature shows promise. The research shows how the detergent and method can decontaminate instruments exposed to infected prions. BioGone was formulated with U.S. EPA Safer Choice ingredients, including a highly concentrated protease enzyme.
Isolate and Sonicate
Utilize a dedicated ultrasonic, even a tabletop model can work, to decrease the potential spread from suspect instruments. BioGone with its safer ingredients and mild alkalinity, replaces highly alkaline detergents and numerous cleaning steps with harsh chemicals, which can degrade surgical devices and potentially harm staff and patients. The solution, however, is not in the detergent alone, but in following the recommended practices. This is where the “recipe” or procedure must be followed to get the very best outcome. To activate the enzymatic detergent, high purity water must be used before immersing the instruments in an ultrasonic cleaner (or water bath), heated to 60°C, for a minimum of 20 minutes. This method must be followed by sterilization. While a four-minute pre-vacuum sterilization cycle showed promise, an 18-minute extended cycle showed the best results after processing.

Do you want to learn more about how this revolutionary product can contribute to patient safety?
Contact us at [email protected], any time, or visit us at Booth #824 at the AORN Global Surgical Conference in Boston April 6th – 8th to see the product for yourself!
Contact us at [email protected], any time, or visit us at Booth #824 at the AORN Global Surgical Conference in Boston April 6th – 8th to see the product for yourself!
Connect with Case Medical on social media for more content!
LinkedIn
FaceBook
Visit us anytime at www.casemed.com to learn more about our products and services. We are here to help. Case Medical is a U.S. EPA Safer Choice Partner of the Year and a Frontrunner in the Chemical Footprint Project. Contact us today to learn more about our certified eco-friendly cleaners and detergents and how we can help your facility’s bottom line with reusable and environmentally preferred products and services.
Kindest Regards,






