Knowledge & Best Practices
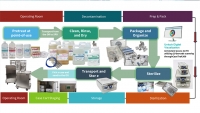
Sterile Processing


Understanding effective management
Embrace Innovation and Technology


Regulatory Requirements
The webinar looks at how FDA conducts inspections of regulated facilities to determine a facility’s compliance with regulations and ultimately reports on observations, violations, and a Corrective and Preventive Actions (CAPA) plan is given. How the Joint Commission evaluates health care facilities is discussed, and what it means to meet ISO international standards.
Register, don't delay
Managing an SPD means a commitment to best-practices, safer alternatives, science-based facts, and continuous improvement. With this webinar we re-start our online educational offerings with CE credits from HSPA and CBSPD. On June 2, 2022, please join us for “Managing Sterile Processing”. There will be two sessions for your convenience, one in the morning and a second session in the evening.
Click to register for 11:00 am EDT Click to register for 6:00 pm EDT
Visit us at www.casemed.com to learn more about our products and how they can help your facility lighten its impact on the environment for the good of us all.
Marcia Frieze and the Case Medical team