What’s Going On?
Off label use of germicides without rinsing, damaged and pitted medical devices, and stripped sterilization containers with white powder residue can all be resolved... I’ve had an opportunity to visit several health care facilities and hopefully provide some ideas for improvement of process and product. As a medical device manufacturer and a U.S. EPA Safer Choice Partner of the Year, our goal is and has been to design safer products, formulate more sustainable cleaners, and educate on best practices and environmentally friendly solutions for instrument processing. Nonetheless when I go into health care facilities, I wonder why so many users still believe that caustic cleaners, standard cycles in cart washers followed by sheeting agents, and especially environmental wipes and germicides designed for surface disinfection, are fine to use for cleaning medical devices! They may be off label and subject to citations.
Consider your safety and that of the patient
Hospitals are communities of ill people; some immunologically suppressed. Safe chemical use includes minimizing exposure to chemicals, proper training, understanding chemical hazards, proper labeling, proper storage and segregation, and proper transport. Knowing and understanding the chemicals you use, their health hazards, their labels and how to control them should an accident occur, is the responsibility of every user. According to Occupational Health for Healthcare Providers, “Many hazardous chemicals are present in healthcare settings, which may pose an exposure risk for healthcare workers, patients, and others.” Chronic exposure to HLDs may cause asthma and asthma-like symptoms or reproductive effects. WARNING: When handling hazardous chemicals, CDC reinforces the use of personal protective equipment including appropriate respiratory equipment. In using disinfectants, the safety of all personnel should be considered.


Cleaning the critical first step
“Cleaning is the necessary first step of any disinfection process. Cleaning removes organic matter, salts, and visible soils, all of which interfere with microbial inactivation. The physical action of scrubbing with detergents and surfactants and rinsing with water removes substantial numbers of microorganisms. If a surface is not cleaned first, the success of the disinfection process can be compromised. Removal of all visible blood and inorganic and organic matter can be as critical as the germicidal activity of the disinfecting agent. When a surface cannot be cleaned adequately, it should be protected with barriers.” https://www.cdc.gov/oralhealth/infectioncontrol/faqs/cleaning-disinfecting-environmental-surfaces.html
What comes first cleaning or disinfecting?
Cleaning alone removes most types of harmful germs (like viruses, bacteria, parasites, or fungi) from surfaces. According to the CDC, surfaces should be cleaned before they are sanitized or disinfected because impurities like dirt may make it harder for chemicals to get to and kill germs. Leaving large amounts of organic matter on surfaces and disinfectants may not be able to kill them. Disinfectants don't necessarily clean surfaces. Germs can hide under dirt and grime and are not affected by disinfectants. Some disinfectants don't disinfect in the presence of dirt. Some disinfectants are fixative that lock in soil. The products used to disinfect are more toxic and usually more expensive than products used to just clean. In fact, failure to clean adequately is the most common failure in the decontamination process.

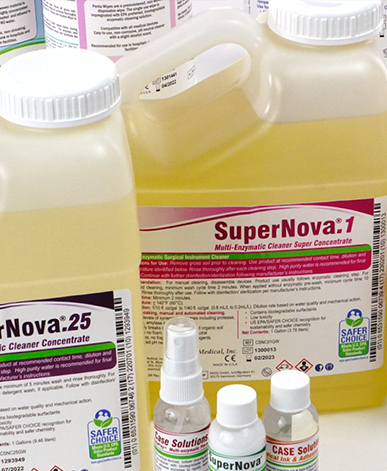
Safer chemical usage
The safety and effectiveness of the product is the responsibility of the manufacturer. While instrument cleaners are not necessarily regulated, at Case Medical, we feel it’s our responsibility to validate our cleaners and chemical solutions with EPA, certify them for safety and effectiveness and display the EPA Safer Choice label on our products to indicate that the products are safer than other items in their class and sustainable for future generations. Case Medical has national GPO agreements, as well as government contracts. Along with a sole source agreement from Vizient under their NovaPlus program and a dual source agreement from Premier for AscenDrive. We are given these contracts based upon clinical preference and cost savings and hope that the sustainable advantages are recognized as well. Our mission is to support best practices, safer patient outcomes, with proven products and services.
Case Medical is a U.S. EPA Safer Choice Partner of the Year and here to help you solve problems with sustainable and validated products and services. Look for the Safer Choice label when purchasing cleaning solutions for your department and for your home.
Visit us anytime at www.casemed.com to learn more about our products and services. We are here to help. Case Medical is a U.S. EPA SAFER CHOICE Partner or the Year for Manufacturer Formulator and recognized in NJ as Innovative Manufacturer of the Year.
Kindest Regards,