HOW WATER SOFTENERS WORK AND WHY THEY FAIL IN THE SPD
 Per USGS, “Nearly 85% of the U.S. is a hard water area.”
Per USGS, “Nearly 85% of the U.S. is a hard water area.”
And water treatment companies are actively promoting water softeners to SPD.
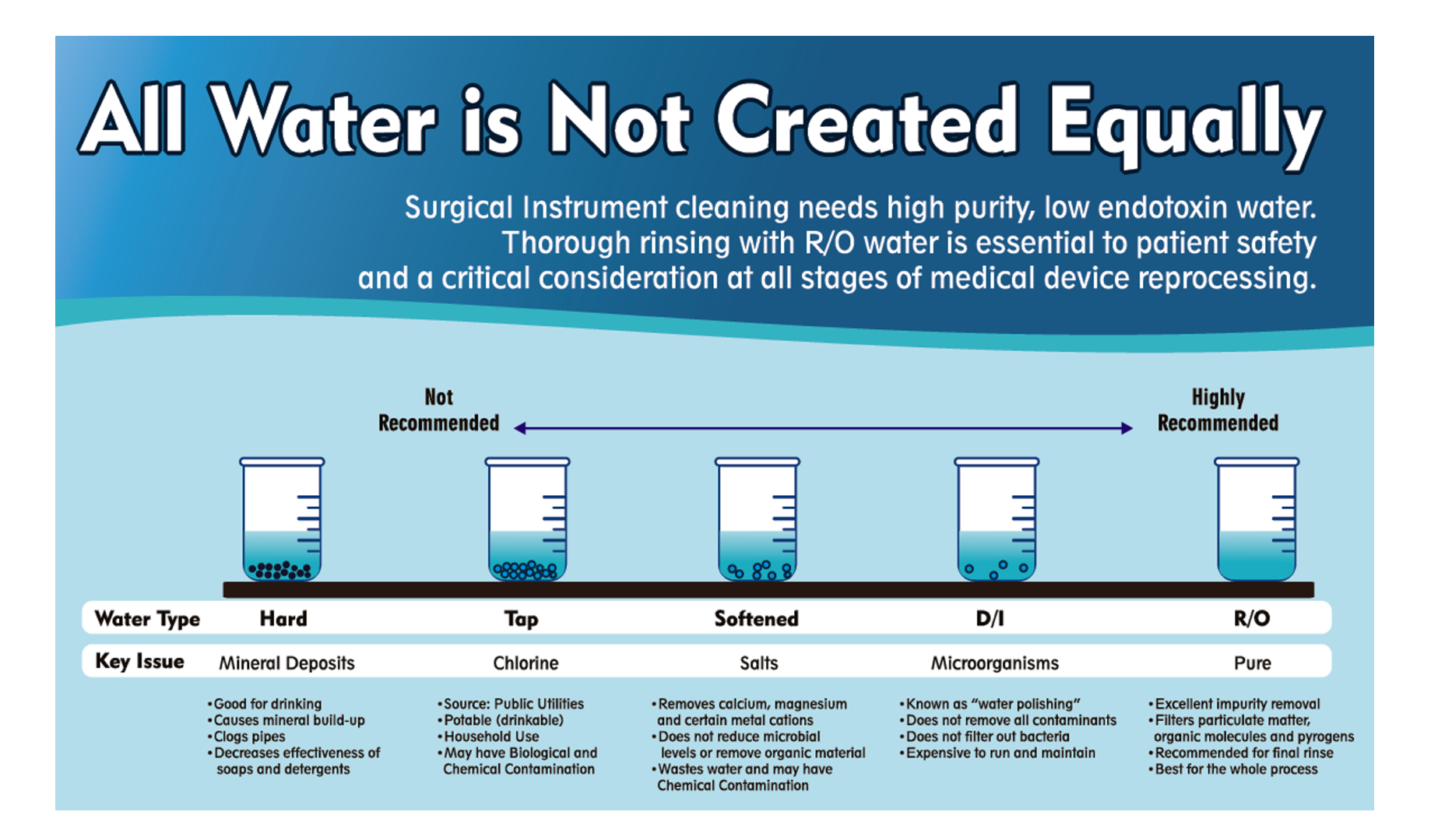
Water softeners work through a process called ion exchange, a typical water-softening system removes calcium and magnesium ions from hard water and replaces them with sodium ions. Filtration solely removes and does not add anything to the water. Water softening adds stuff you may not want. While water softeners may remove mineral ions, they do not remove contaminants such as bacteria or heavy metals like lead, mercury, or even iron.
 Remember, RO and DI water systems are currently available for treating water used for instrument processing. These systems use filtration or resin instead of salt. Then, why add a water softener as recommended by some suppliers? On a personal note, should we drink softened water or even shower with it? Did you ever feel the slimy residue on your skin after bathing? Consider the effect on your valuable instrumentation.
Remember, RO and DI water systems are currently available for treating water used for instrument processing. These systems use filtration or resin instead of salt. Then, why add a water softener as recommended by some suppliers? On a personal note, should we drink softened water or even shower with it? Did you ever feel the slimy residue on your skin after bathing? Consider the effect on your valuable instrumentation.
Is any sodium in water a benefit?
Softening “adds about 750 milligrams of sodium to each gallon of water,” * effectively turning tap water into saline solution. The amount of sodium added by a water  softener is linearly related to the number of hardness minerals being reduced. For every milligram of hardness in the water, the softener releases two milligrams of sodium.
softener is linearly related to the number of hardness minerals being reduced. For every milligram of hardness in the water, the softener releases two milligrams of sodium.
*Ref. Scientific American - Chuck Wight, a chemistry professor at the University of Utah, September 24, 2001
Should Water Softeners Be Used for Instrument Processing?
We do not think so. Water softeners can contribute to corrosion. Saline and hydrogen peroxide applied to metal causes a rusty surface sometimes valued as a patina to make the new metal into aged-looking pieces of art. That may be desirable if you want to put a patina on a metal object as a garden feature or a work of art. However, a rusty patina or corrosion on surgical devices has no place in the SPD or the OR.
If you would like to experiment at home, visit this website to experience this process firsthand.
Instructables.com - How-to-Turn-Metal-Rusty
Strong Chlorides Cause Pitting Corrosion in Stainless Steel and Aluminum
Many types of stainless-steel alloys will suffer extreme pitting corrosion when exposed to environments that are rich in salt. Most surgical devices undergo a passivation process to render the material non-corrosive. Exposure to salt accelerates or directly contributes to corrosion.

Case Medical, a manufacturer of sealed containers
We have learned a thing or two about how metals can rust. We’ve learned how the combination of saline residue from water softeners followed by hydrogen peroxide sterilization can corrode our products. We strongly advise that when you process instruments and any aluminum container you find a better water source, most importantly for low temperature sterilization.

In conclusion, water filtration is a better choice. Reverse osmosis which pushes water through a membrane eliminating dissolved solids, salts, and even microorganisms from the water contributes to better outcomes. That is why Case Medical offers RO water systems for the highest quality critical water available for instrument processing.
To learn more about safe and effective water treatment options from Case Medical contact us at [email protected] or CLICK HERE for more information.





