While some would say that all instrument cleaners are more or less the same, in reality the products you choose can be as different as using a mallet or a brush to uncover ancient pottery. Formulating cleaners and detergents that are effective while maintaining the integrity of your surgical instruments and containers depends upon two things: 1) scientific understanding and 2) a commitment to using high quality, safer chemical ingredients.
When you evaluate the information on your detergent labels, consider the pros and cons of the elements discussed below.
Decoding: Enzymes
Enzymes break down targeted organic substances (proteins, fats, starches, etc.). They significantly decrease the activation energy required, while increasing the speed of chemical reactions. In some cases, enzymes can make a chemical reaction millions of times faster than it would have been without them. This is true of digestion in our bodies and instrument processing in our Sterile Processing Departments.
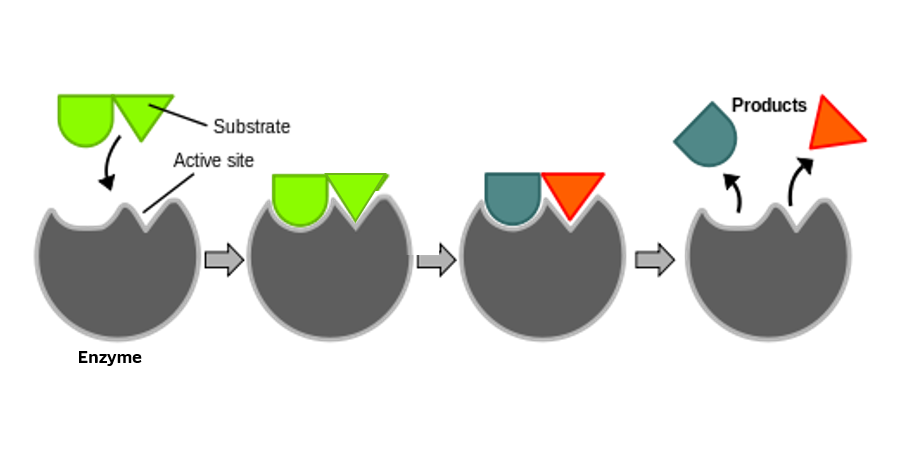
Enzymes are highly effective, a natural, targeted component of certain cleaners. Often used for wastewater treatment, enzymes are safe for disposal into the wastewater system. Enzymes and surfactants do the heavy lifting of cleaning. As with all cleaning processes, correct machine settings and adequate rinse cycles also contribute to the best outcomes. (Want to learn more? Register for our CE webinar, "Enzymes and Their Actions.")

Decoding: Silicates
You may even find that your detergent contains silicates. What are silicates? Silicate minerals are rock-forming minerals and make up approximately 90 percent of Earth's crust. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts. Silicates, when added to detergents, have the effect of raising the alkalinity.
Silicates are often used in detergents, paper, water treatment, and construction materials and have abrasive qualities. If you observe discoloration (white-gray, yellowish brown, or bluish purple) on your instruments, it may be silicate deposits from insufficient rinsing or corrosion.
Decoding: Aluminum Friendly
Detergents labeled as “Aluminum Friendly” or "Aluminum Safe" are actually highly alkaline, caustic, and damaging to anodized aluminum containers and even your surgical devices. Alkaline detergents are found on the higher end of the pH scale, with water as neutral at 7, and lower values representing acidic substances. While alkaline cleaners remove soil, they can create more problems than they solve.
In fact, some “aluminum friendly” detergents have a pH as high as 14. Highly alkaline cleaners can cause discoloration, corrosion, and material erosion, characterized by the presence of a white, powder-like coating on anodized aluminum and pitting and rusting on instruments. A simple demonstration using three solutions, pH levels 7.6, 11, and 14, demonstrates the effect of alkaline solutions on anodized aluminum coupons.
In fact, some “aluminum friendly” detergents have a pH as high as 14. Highly alkaline cleaners can cause discoloration, corrosion, and material erosion, characterized by the presence of a white, powder-like coating on anodized aluminum and pitting and rusting on instruments. A simple demonstration using three solutions, pH levels 7.6, 11, and 14, demonstrates the effect of alkaline solutions on anodized aluminum coupons.
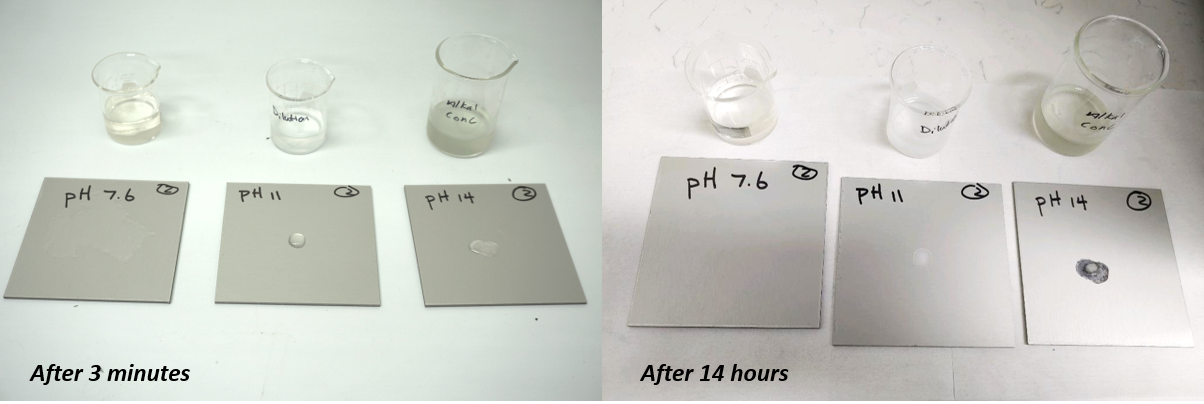
At just three minutes, the sample exposed to a pH 14 solution shows corrosion (white area). After 14 hours of continuous exposure, the same coupon shows corrosion and metal flaking producing a black appearance, and the pH 11 solution is causing corrosion on the center coupon.
Caustic formulations also result in reduced use life for devices treated, requiring repair, refurbishing, or replacement. Most concerning, alkaline cleaners can cause severe skin burns and eye damage to the user.

Sorting through the messages
As with most decisions, choosing instrument chemistries requires sorting through conflicting messages from multiple sources. Do your research, call on people you trust, and learn the science so you can critically evaluate the messages you hear. Your choices do matter.
They matter to the personnel who have to use these chemicals in their daily work and to the patients whose bodies are susceptible to any retained residue or soil.






