The Dirty Truth
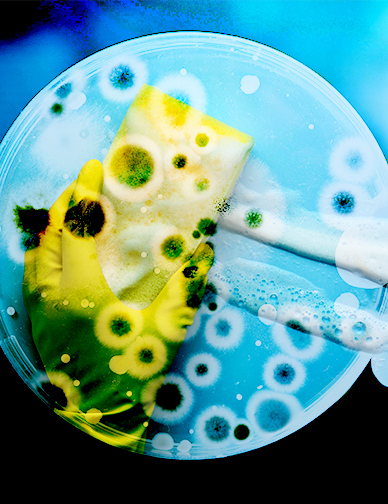
There are 54 billion bacterial cells on a single cubic centimeter of the average kitchen sponge. They are breeding grounds for all types of germs and bacteria. A common sponge’s spatial partitioning – the way it’s divided into different sectors of various sizes with a porous absorbent surface – caters to bacteria that prefer isolated environments and those that prefer to be around other organisms as well, making it the best of both worlds for microbial communities. When it comes to the first step of endoscope processing, the initial bedside cleaning of the device, using a sponge, may not be the best practice. “A single sponge can harbor a higher number of bacteria than there are people on Earth.” Despite these risks, many people in the endoscopy suite continue to use sponges when there is a better solution available...

ECRI Health Hazards
The ECRI Institute has identified the mishandling of flexible endoscopes after disinfection as the 5th leading health technology hazard in their 2019 report and processing endoscopes was typically one of the top health hazards for several years before. It’s not just cleaning! Improper handling and storage practices have the potential to recontaminate previously disinfected scopes, thereby heightening the risk of patient infections. While ECRI continues to list concerns about cleaning medical devices, ECRI has not included endoscope processing in their most recent publication.
Public Health Concern
Just this week, the issue of inadequate cleaning of endoscopes became front and center again. According to a report on October 11th, there is growing concern surrounding Vanderbilt Health’s endoscopy clinic and its current decontamination measures. Vanderbilt Health potentially exposed an unspecified number of patients to HIV and hepatitis B and C. One patient notified of her potential exposure during a routine colonoscopy stated, “I am worried about everyone and everything and how I can continue my life in a normal way”...wondering if I do have it.” While a representative from Vanderbilt Health claims less than 4% of endoscopy patients who visited the clinic within the last six months risk exposure, improper cleaning continues to pose a threat for patients.

Pre-Treatment at Bedside
We know that pre-cleaning makes a huge difference in the effectiveness and efficiency of reprocessing medical devices especially when a validated pretreatment cleaner is applied at point-of-use. This is of value when a multi-enzymatic pre-treatment is used in the endoscopy suite. Cleaning and disinfection of flexible endoscopes presents a significant challenge to reprocessing personnel because of the numerous critical steps that need to be followed. In addition, there is pressure to quickly turn around the endoscope so that it is ready for the next patient.
The Ideal Choice
Enzymes are catalysts that speed reactions and those in multi-enzymatic detergents are little different than the digestive enzymes in our gut to digest food. In a similar way, enzymes break down organic contaminants and bodily soils on surgical devices. Endoscopic procedures, while using minimally invasive devices, are exposed to and collect a full range of bodily soils that must be removed for the well-being of the next patient and for the staff. The importance of pre-cleaning endoscopes at the point of use is spelled out by many organizations including, AORN, SGNA, HSPA, and AAMI standards ST79 and specifically ST91.


Case Medical to the Rescue
This is where Case Medical comes to the rescue with multi-enzymatic cleaners, single-use wipes, detergents, instrument brushes, and the Fast Foamer device designed specifically for the endoscopy suite. Consider our Endoscopy Bedside Kit with pre-measured 250 ml of PentaZyme for suctioning the inner channel and 3 disposable, white, lint-free PentaWipes for sequential exterior cleaning of the scope. Think of the last wipe in the bedside kit as the final indicator of cleanliness. Just the right amount of multi-enzymatic detergent, for cleaning the inside and outside of the scope. Start the cleaning process with Case Medical’s Endoscopy Bedside Kit to avoid bioburden buildup and dried soil that can make subsequent cleaning steps a challenge. Then use the Fast Foamer available from Case Medical for decontamination using Pentazyme.
Keeping it all Together
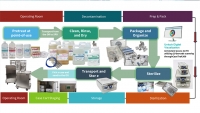
Endoscopes require that the user follow numerous steps (sometimes over 100) for effective decontamination prior to disinfection or sterilization. With so many steps to follow, you need a helping hand, or a digital assistant to keep you on track and error-free. Case Medical developed CaseTrak360®, our robust asset management software, and its companion Endo Module which identifies all endoscope processing steps. Further, it maintains records of your instrumentation in real-time and provides access to IFUs, as well as staff’s competency certifications. So, when The Joint Commission audits your facility, you'll be ready with complete documentation.

Connect with Case Medical on social media for more content!
LinkedIn
FaceBook
Visit us anytime at www.casemed.com to learn more about our products and services. We are here to help. Case Medical is a U.S. EPA SAFER CHOICE Partner or the Year for Manufacturer Formulator and recognized in NJ as Innovative Manufacturer of the Year.
Kindest Regards,