From Tradition-Driven Practices to Standardized, Simplified, and Safer Systems
How often do we hear, “We’ve always done it this way. It’s worked before — why change now?”
Healthcare no longer operates in yesterday’s world. The Industrial Revolution has given way to the Digital Revolution, transforming how we diagnose, treat, and operate. Minimally invasive procedures now rely on sophisticated endoscopes and robotic instruments — devices that demand meticulous, step-by-step reprocessing and strict adherence to IFUs to ensure safety, performance, and longevity. In this environment, clarity is critical. Instructions must be accessible. Alerts must be timely. Prompts must be intuitive. Safe, consistent practice depends on it. Technology has changed. The risks have changed. Our mindset must change too. It’s time to put the myths to rest.


Simple Solutions to Replace Old Habits
Overloaded Trays: A Risk We Can No Longer Ignore
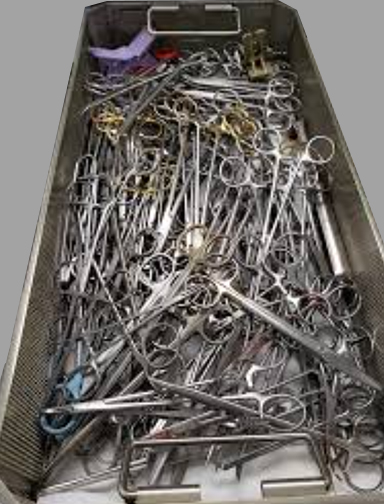

Think Solutions
Standardizing intuitive, validated products reduces variation, lowers errors, and protects patients. When products are designed to work together, complexity drops — and confidence rises. Case Medical provides that solution: SteriTite® reusable sealed container systems compatible across sterilization modalities; Case Solutions® multi-enzymatic and non-enzymatic chemistries engineered for effective cleaning with a thorough rinse; MediTray® instrument organization systems that reduce tray clutter and instrument damage; durable Case Carts that protect and streamline transport; and CaseTrak360® for real-time tracking, documentation, and workflow visibility. Together, they create one standardized system designed for consistency, compliance, and measurable performance.
Ready to reduce variability and strengthen safety? Partner with Case Medical to evaluate your processes and implement a standardized system that delivers consistent results.






